31 July 2006
 |
| Cross-well seismic difference tomogram of the Frio Brine project shows the CO2 plume. |
Results from a field test on CO2 sequestration in an old brine-filled oil reservoir suggest that the mixture of CO2 and brine dissolves minerals in the rock walls, including carbonate, that could lead to pathways in the rock through which the gas could escape.
In a paper published in the July edition of Geology, the researchers in the Frio Brine Pilot also note the potential for the mobilization of toxic trace metals and toxic organic compounds.
The Frio Brine Pilot was the first test of closely monitored CO2 injection in a brine formation in the United States, and was funded by the Department of Energy (DOE) National Energy Technology Laboratory (NETL) under the leadership of the Bureau of Economic Geology (BEG) at the Jackson School of Geosciences, The University of Texas at Austin, with major collaboration from GEO-SEQ, a national lab consortium led by Lawrence Berkeley National Laboratory (LBNL).
The researchers injected 1,600 metric tons of CO2 1,500 meters down into a sandstone site representative of a target for large-volume storage. The sandstones of the Oligocene Frio Formation are part of a thick, regionally extensive sandstone trend that underlies a concentration of industrial sources and power plants along the Gulf Coast of the United States.
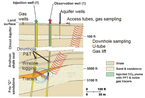 |
| Monitoring strategy at Frio. |
The team then measured and monitored the CO2 plume using a diverse suite of technologies in three intervals: the injection zone, the area above the injection zone, and the shallow near-surface environment.
Each monitoring strategy used a preinjection and one or more postinjection measurements. Wireline logging, pressure and temperature measurement, and geochemical sampling were also conducted during injection, and at follow-up intervals subsequent to the injection.
While the sequestration to-date has been successful—there have been no detected CO2 leakages—the researchers conclude in their latest published assessment of on-going findings and analysis that the chemistry of the process might prove problematic.
Fluid samples obtained from the injection and observation wells before CO2 injection showed a Na-Ca-Cl–type brine with 93,000 mg/L total dissolved solids (TDS) at near saturation with CH4 at reservoir conditions.
Following CO2 breakthrough, samples showed sharp drops in pH (6.5–5.7), pronounced increases in alkalinity (100–3,000 mg/L as HCO3) and Fe (30–1,100 mg/L), and significant shifts in the isotopic compositions of H2O, dissolved inorganic carbon (DIC), and CH4.
Geochemical modeling indicates that brine pH would have dropped lower but for the buffering by dissolution of carbonate and iron oxyhydroxides.
This rapid dissolution of carbonate and other minerals could ultimately create pathways in the rock seals or well cements for CO2 and brine leakage. Dissolution of minerals, especially iron oxyhydroxides, could mobilize toxic trace metals and, where residual oil or suitable organics are present, the injected CO2 could also mobilize toxic organic compounds.
Environmental impacts could be major if large brine volumes with mobilized toxic metals and organics migrated into potable groundwater.

